How do genetic variants result in
altered brain development and epilepsy?
|
|
A recent explosion in gene discovery has revealed hundreds of genes associated with intellectual disability and epilepsy.
There is a critical need to better understand how these gene variants cause disease in order to develop novel therapeutic strategies. |
RESEARCH
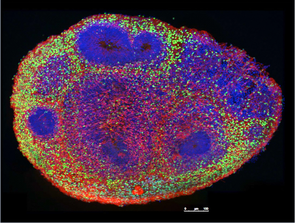
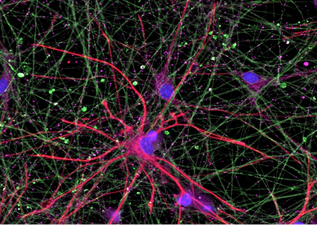
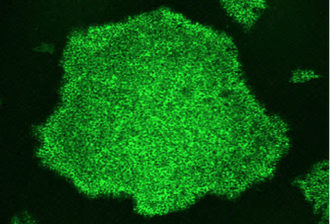
Organoid models of mTORopathiesPathogenic genetic variants in the mechanistic target of rapamycin (mTOR) pathway can cause mTOR hyperactivity, resulting in brain malformations and epilepsy. STRADA is an upstream regulator of the mTOR pathway, and loss of STRADA causes mTOR hyperactivation, and PMSE (polyhydramnios, megalencephaly, and symptomatic epilepsy) syndrome. We use human stem cell models, two- and three-dimensional neuronal cultures, multiple-electrode array recordings, and calcium imaging to determine how pathogenic variants in STRADA that result in mTOR pathway hyperactivation alter cell fate decision in early cortical development and interneuron development and cause brain malformations and epilepsy.
|
Novel therapies for
|
SCN1B-related developmental and epileptic encephalopathyBiallelic pathogenic variants in the SCN1B gene cause a severe developmental and epileptic encephalopathy (DEE) that can have clinical features shared with Dravet Syndrome. In collaboration with Dr. Lori Isom and Dr. Jack Parent, we differentiate patient-derived stem cells into neuronal cultures in order to explore how pathogenic variants in SCN1B cause neuronal dysfunction and epilepsy.
|
THE TEAM
We believe that science is best advanced by
a diverse team working in an inclusive and collaborative environment
a diverse team working in an inclusive and collaborative environment
Lab AlumniDaniel Klarr, 2016
Matthew Parent, summer 2016 Pooja Patel, summer 2017 Trevor Glenn, summer 2016 and summer 2017 Anand Chandrasekhar, 2017-2018 Alexandra (Lexie) Streicher, summer 2018 and summer 2019 Jordan Safran, 2017-2019 Monica Lee, summer 2020 Preethi Swaminathan, 2016-2020 Shivanshi Vaid, 2018-2022 Anna Loughman, 2019-2022 Daniel Jaklic, 2022-2023 Grace Randolph, summer 2023 Sahej Kohli, 2022-2024 |
|
PUBLICATIONS
- Dang, L.T., Parent, J.M. “Genetic Epilepsy Modeling with Human Pluripotent Stem Cells.” In: Models of Seizure and Epilepsy, Second Edition. Edited by Pitkänen, A., Buckmaster, P.S., Galanopoulou, A.S., and Moshe, S.L. Elsevier publishing, 2017, 247-260.
- Tidball, A.M., Dang, L.T., Glenn, T.W., Kilbane, E.G., Klarr, D.J., Margolis, J.L., Uhler, M.D., Parent, J.M. Rapid Generation of Human Genetic Loss-of-Function iPSC Lines by Simultaneous Reprogramming and Gene Editing. Stem Cell Reports, 2017; 9:725-31.
- Tidball, A.M., Swaminathan, P., Dang, L.T., Parent, J.M. Generating Loss-of-function iPSC Lines with Combined CRISPR Indel Formation and Reprogramming from Human Fibroblasts. Bio-protocol, 2018; 8(7): e2794.
- Frasier, C.M., Zhang, H., Offord, J., Dang, L.T., Auerbach, D.S., Shi, H., Chen, C., Goldman, A., Eckhardt, L.L., Bezzerides, V.J., Parent, J.M., Isom, L.L. Channelopathy as a SUDEP biomarker in Dravet Syndrome patient-derived cardiac myocytes. Stem Cell Reports, 2018; 11:626-634.
- Dang, L.T., Quinonez, S.C., Becka, B.R., Isom, L.L., Joshi, S.M. Dramatic improvement in seizures with phenytoin treatment in an individual with refractory epilepsy and a SCN1B variant. Pediatric Neurology, 2020; 108:121-122.
- Dang, L.T., Glanowska, K.M., Iflland, P.H., Barnes, A.E., Baybis, M, Liu, Y., Patino, G., Vaid, S., Streicher, A.M., Parker, W., Kim, S., Moon, U.Y., Henry, F.E., Murphy, G.G., Sutton, M.A., Parent, J.M., Crino, P.B. Multimodal analysis of STRADA function in brain development. Frontiers in Cellular Neuroscience, 2020; 14:122.
- Dang, L.T., Vaid, S., Lin, G., Swaminathan, P., Safran, J., Loughman, A., Lee, M., Glenn, T., Majolo, F., Crino, P.B., Parent, J.M. STRADA-mutant human cortical organoids model megalencephaly and exhibit delayed neuronal differentiation. Developmental Neurobiology, 2021; 00:1-14. doi: 10.1002/dneu.22816 PMID: 33619909
LAB NEWS
|
5/1/23 Welcome, Dr. Tong Pan to the lab! We are excited to have Tong as the lab's first postdoc to join! She is a neurologist and physician-scientist with a background in novel receptors/ion channels that mediate thermosensation using C. elegans as a model. Tong is excited to learn about using stem cell derived cerebral organoid models for neurodevelopmental diseases. |